Bridging therapy is a temporary treatment given to patients undergoing CAR-T cell therapy to control their cancer while waiting for their personalized CAR-T cells to be manufactured and infused. Since CAR-T therapy is often used for relapsed or refractory (R/R) blood cancers, such as Large B-Cell Lymphoma (LBCL), Acute Lymphoblastic Leukemia (ALL), and Multiple Myeloma (MM), these cancers can progress rapidly without interim treatment.
Why Is Bridging Therapy Needed?
Patients undergoing CAR-T therapy usually have fast-growing cancers that can worsen in just a few weeks. The CAR-T cell production process typically takes time, during which cancer may progress.
Bridging therapy is used to:
- Prevent Disease Progression – Keeps cancer from spreading while waiting for CAR-T infusion.
- Maintain Patient Stability – Ensures patients remain well enough to receive CAR-T therapy.
- Optimize CAR-T Effectiveness – Reducing the cancer burden may improve CAR-T success.
Bridging Therapy in Academic CAR-T: Is It Necessary?
In academic CAR-T therapy, where the wait time is only 10-12 days, bridging therapy is not always required. However, in cases where a patient has rapidly progressing disease, oncologists may still recommend short-term bridging therapy to stabilize the condition before infusion.
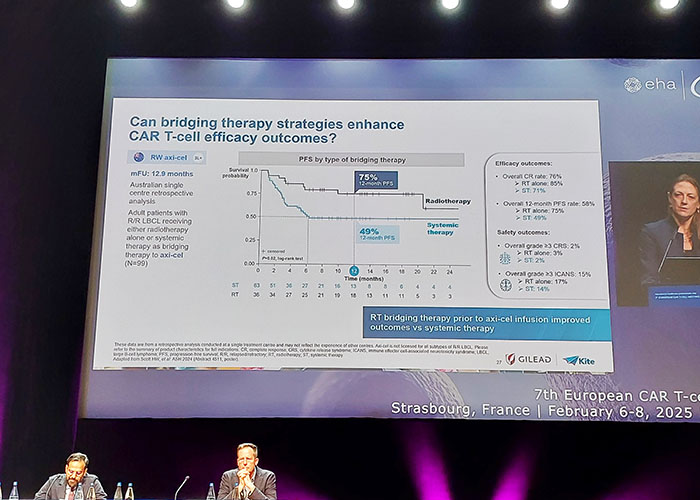
7th European CAR-T conference. Strasbourg, France 2025.
Types of Bridging Therapy:
Depending on the patient’s cancer type and urgency, different therapies may be used:
- Chemotherapy – Low-dose regimens to control tumor growth.
- Immunotherapy – Monoclonal antibodies (e.g., rituximab, daratumumab).
- Targeted Therapy – Drugs like ibrutinib or venetoclax to slow cancer progression.
- Radiotherapy – In select cases, radiation can shrink tumors quickly.
- Steroids – Used in some lymphomas and leukemias to reduce tumor burden.
When Is Bridging Therapy Not Needed?
- If the disease is stable and the CAR-T cell production time is short (10-12 days in academic CAR-T), many patients can go directly to lymphodepleting chemotherapy before infusion.
- Some treatments used in bridging therapy can suppress T cells, which may impact the effectiveness of CAR-T. Doctors carefully weigh the risks before deciding on bridging therapy.
Conclusion:
Bridging therapy is an essential tool for many CAR-T patients, particularly when the wait time is long in commercial CAR-T production. However, in academic CAR-T programs, where manufacturing is faster (10-12 days), many patients can proceed without bridging therapy unless their disease is progressing aggressively.
Prof. Arnon Nagler from Sheba hospital speaks about commercial and Academic CAR-T
Publication date: March 2025










