Chimeric Antigen Receptor T-cell (CAR-T) therapy has emerged as a promising treatment for multiple myeloma (MM), particularly for patients who have exhausted standard treatment options. This article outlines current eligibility criteria, timing considerations, and evolving trends in CAR-T therapy for MM.
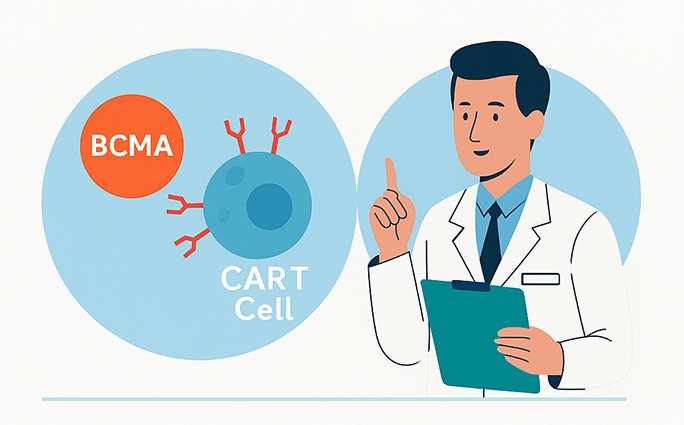
What Is CAR-T Therapy?
CAR-T therapy involves collecting a patient's T cells, genetically modifying them to express chimeric antigen receptors (CARs) that target specific proteins on cancer cells, and then reinfusing them into the patient to attack the cancer.
In MM, the primary target is B-cell maturation antigen (BCMA), a protein commonly found on myeloma cells. FDA-approved CAR-T therapies for MM include idecabtagene vicleucel (Abecma) and ciltacabtagene autoleucel (Carvykti).
In addition, numerous academic CAR-T programs are were developed to treat multiple myeloma, with success rates similar to commercial therapies.
Who Is Eligible for CAR-T Therapy?
Eligibility for CAR-T therapy in MM is determined by several factors:
- Disease Status: Patients with relapsed or refractory multiple myeloma who have failed 2–3 different lines of treatment are typically considered.
- Performance Status: Patients should have a good performance status, usually an ECOG score of 0–2. In simpler terms: the patient should be in good physical condition—able to walk, eat, and bathe independently.
- Organ Function: Adequate heart, liver, lung, and kidney function is required. This is usually assessed through blood tests.
- Age: There is no strict upper age limit. Patients up to 91 years old have been successfully treated.
- Infection Status: Active infections must be resolved before starting therapy.
Note: Eligibility criteria may vary between institutions and clinical trials.
When Is CAR-T Therapy Recommended?
CAR-T therapy is generally considered for MM patients who:
- Have relapsed or refractory disease after multiple lines of therapy
- Are not candidates for autologous stem cell transplantation
- Have adequate organ function and performance status
Recent studies suggest that using CAR-T therapy earlier in the treatment course may improve outcomes. Ongoing clinical trials are evaluating its use in earlier treatment stages.
Considerations and Side Effects
While CAR-T therapy offers new hope for many patients, it is also associated with some potential side effects:
- Cytokine Release Syndrome (CRS): A systemic inflammatory response that may cause fever, low blood pressure, and organ dysfunction.
- Neurotoxicity: Symptoms can include confusion, seizures, or altered mental status.
These side effects are typically manageable with timely medical care.
Accessing CAR-T Therapy in Israel
In Israel, CAR-T therapy for multiple myeloma is available at several leading medical centers. Patients interested in this treatment should first consult with their hematologist or oncologist. If CAR-T is recommended, you are welcome to apply through our platform—we’ll help identify the best available treatment option and guide you through the process to make it as clear and stress-free as possible.
Publication date: May 1, 2025.
Sources
1. American Cancer Society – CAR T-cell Therapy for Multiple Myeloma
2. Massachusetts General Hospital – CAR T-Cell Therapy for Multiple Myeloma
3. CARTHope – Patient Eligibility for CAR-T Therapy
4. National Cancer Institute – FDA Approves Carvykti CAR T-Cell Therapy for Multiple Myeloma
5. NIH / PubMed Central – CAR T-Cell Therapy for Patients with Multiple Myeloma










