Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS) is a neurological side effect that can occur following chimeric antigen receptor (CAR) T-cell therapy. It typically manifests within days to weeks after treatment and presents with symptoms ranging from mild confusion and language disturbances to severe cases involving seizures or cerebral edema.
The good news is that ICANS is generally reversible. Most patients experience a full recovery within 3 to 8 weeks, especially with prompt and appropriate management. Treatment often involves supportive care and may include corticosteroids to reduce inflammation. However, in rare instances, ICANS can be severe and potentially life-threatening, underscoring the importance of early detection and intervention.
Ongoing research aims to better understand ICANS and develop strategies to prevent and manage this condition more effectively. This includes identifying patients at higher risk and refining CAR T-cell therapies to minimize adverse effects while maximizing therapeutic benefits. In summary, while ICANS is a concerning potential side effect of CAR T-cell therapy, it is usually reversible with timely and appropriate medical care.
What is ICANS?
ICANS, or Immune Effector Cell-Associated Neurotoxicity Syndrome, is a temporary neurological condition that may occur after CAR-T cell therapy. It can cause symptoms such as confusion, difficulty speaking, or in more serious cases, seizures. While the symptoms can be alarming, ICANS is usually reversible with proper medical care and monitoring.
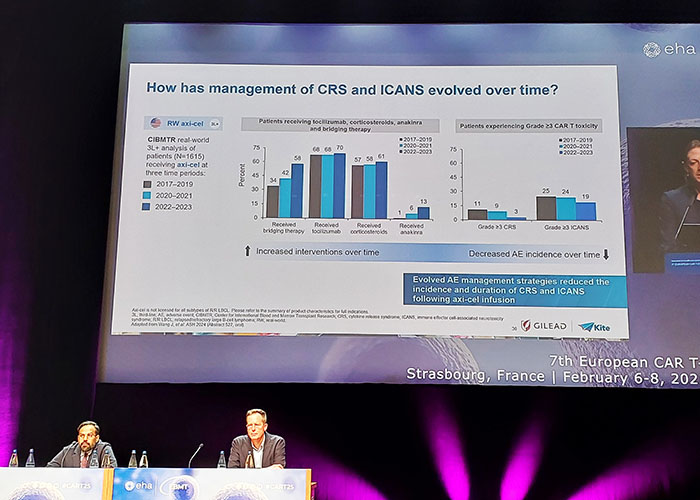
Photo: European CAR-T conference 2025. Strasbourg, France.
Publication date: April 2025
Sources:
ncbi.nlm.nih.gov
ncbi.nlm.nih.gov
frontiersin.org
uptodate.com
ashpublications.org
ncbi.nlm.nih.gov
frontiersin.org










