Advancements in CAR-T Therapy for Multiple Myeloma at Hadassah Medical Centre 2023
Hadassah Hebrew University Medical Centre in Jerusalem continues to lead the field in CAR-T cell therapy with its innovative approaches for treating multiple myeloma and light chain amyloidosis. The institution produces the CAR-T cell product HBI0101, developed to target BCMA on cancer cells, as part of a registered clinical trial. This program highlights cutting-edge methods and patient-centric advancements in CAR-T manufacturing and application.
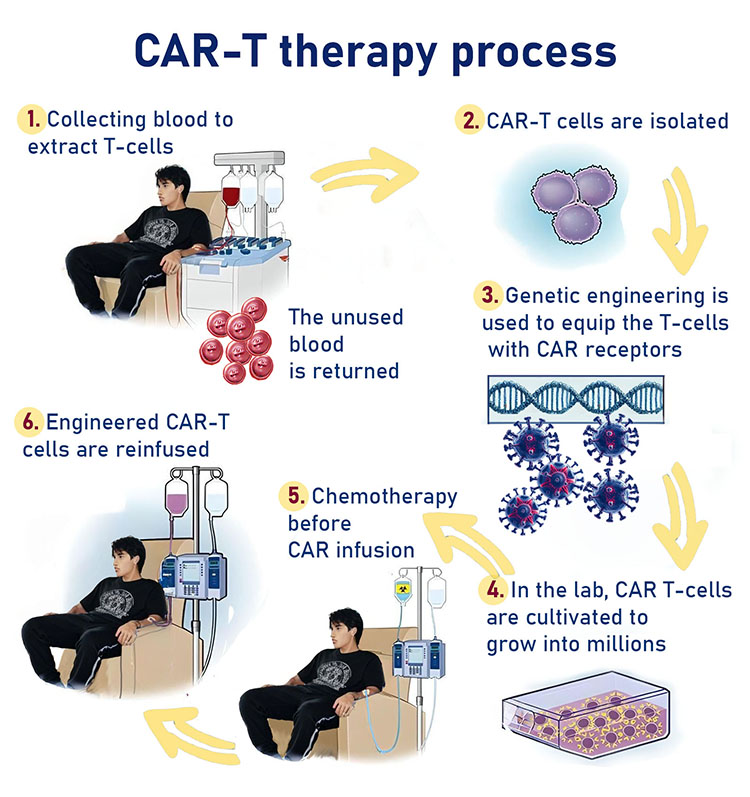
CAR-T Patient Treatment Process
The journey begins with apheresis, a specialized blood collection procedure to isolate T-cells. Conducted at the Bone Marrow Transplantation Department, this step typically lasts 2–4 hours. Once collected, the cells are either processed immediately or cryopreserved for future use. Manufacturing takes place in Hadassah’s GMP-certified facility, where the T-cells are stimulated, genetically engineered to express BCMA-targeting receptors, and expanded to reach therapeutic levels.
Currently, patients receive freshly prepared CAR-T cells, but Hadassah is transitioning to cryopreserved products. This shift will increase flexibility, allowing treatments to adapt to logistical and clinical needs. The final product undergoes stringent safety and quality tests before being infused into the patient under close medical supervision.
Expertise and Team Collaboration
Hadassah’s multidisciplinary team ensures seamless coordination between clinical and manufacturing stages. Led by Prof. Polina Stepensky, the team comprises highly skilled professionals in quality assurance, research, and manufacturing. Patients are carefully evaluated for eligibility, and their health is monitored throughout the process, ensuring the highest standard of care.
Challenges in CAR-T Production
Despite its success, CAR-T manufacturing presents challenges such as achieving sufficient cell proliferation and maintaining sterility. Hadassah addresses these issues through vigilant monitoring and quick interventions when production hurdles arise. Plans to automate parts of the manufacturing process aim to reduce costs, enhance efficiency, and improve the durability of CAR-T cells in patients.
Expanding Access and Capabilities
Hadassah’s facility currently produces up to four CAR-T therapies per month but is preparing to scale operations. Additional production suites and the use of cryopreserved CAR-T cells will increase capacity and allow for international transport, expanding access to patients from around the world.
Future CAR-T Goals
The hospital’s next steps include shortening manufacturing times and reducing the length of patient hospitalizations. By refining production techniques, Hadassah aims to create CAR-T cells with enhanced longevity, offering better outcomes for patients. Cryopreserved products will further streamline treatment by eliminating the need for lengthy hospital stays, especially for international patients.
Conclusion
Hadassah Medical Centre’s CAR-T program exemplifies innovation and dedication to patient care. Through continuous advancements in technology and process optimization, the hospital is poised to deliver life-changing treatments to more patients locally and internationally.
Source:
Myeloma Patients Europe










