Overview by Dr. Francesca Lorraine Lim discussed the development of CAR-T cell therapy at Singapore General Hospital (SGH). The information was shared during the 6th EBMT conference in 2024.
Progress in CAR-T therapy Key Milestones:
- 2017 - Initial Recognition and Resource Allocation:
o After FDA approval of commercial CAR-T therapies, Singapore allocated resources to develop clinical teams and trial units. - 2019 - Participation in Clinical Trials:
o SGH participated in pivotal clinical trials, gaining experience with the logistics and patient selection for cell and gene therapies. - March 2021 - Regulatory Approval:
o Health Science Authority approved the use of Kymriah for third-line treatment.
o This period also saw the establishment of relevant regulations. - March 2022 - Academic CAR-T Program Initiation:
o SGH launched an academic CAR-T program to address accessibility concerns.
o The Minister of Health formed a team to develop national policies for cell therapy programs. - March 2023 - Approval of Yescarta:
o Yescarta received approval for third-line use.
o SGH established a national GMP (Good Manufacturing Practice) facility.
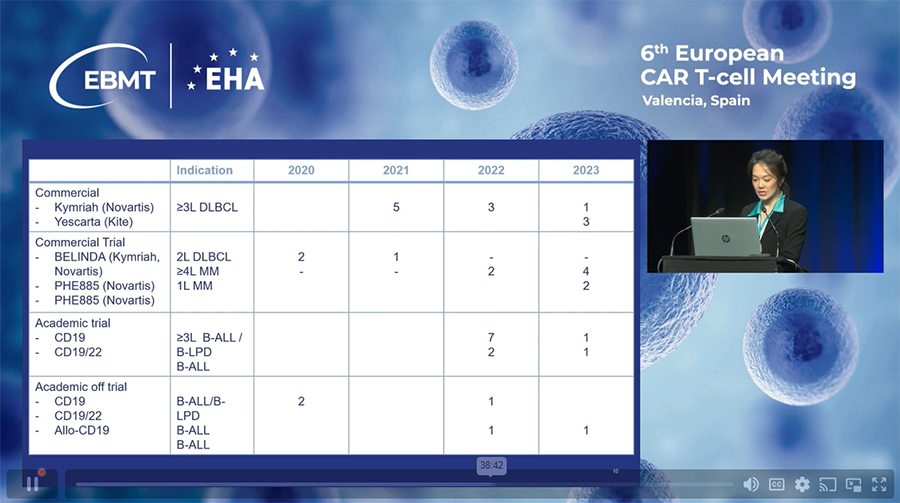
Program Development:
- Integration with Stem Cell Transplant Program:
o SGH's CAR-T cell program is integrated into its existing stem cell transplant program.
o The program was renamed to the Hematopoietic Cell Therapy and Transplant Program. - In-house Capabilities:
o SGH has accredited apheresis facilities and comprehensive processing and storage capabilities.
o The hospital started using commercial CAR-T therapies in 2021, alongside its academic program launched in 2022.
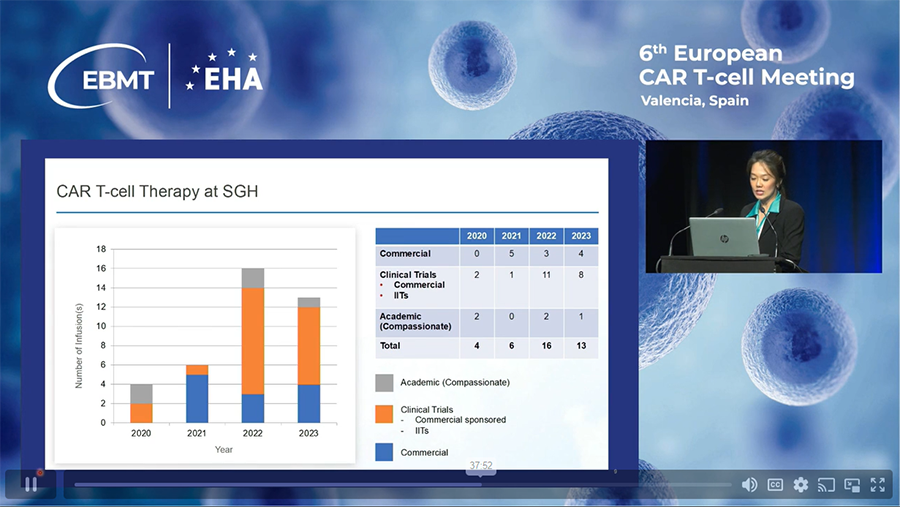
Current Clinical Program:
- Commercial and Academic CAR-T Therapies:
o SGH offers Kymriah and Yescarta for third-line treatment.
o Participation in Novartis clinical trials for BCMA CARs.
o Academic CAR-T program includes CD19 and dual construct autologous CAR-T therapies, manufactured using point-of-care project machines. - Case-by-case Eligibility:
o Patients not meeting academic trial criteria may still receive treatment based on individual assessments and discussions with the clinical team.
Total cases as of December 2023: 39 cases.










