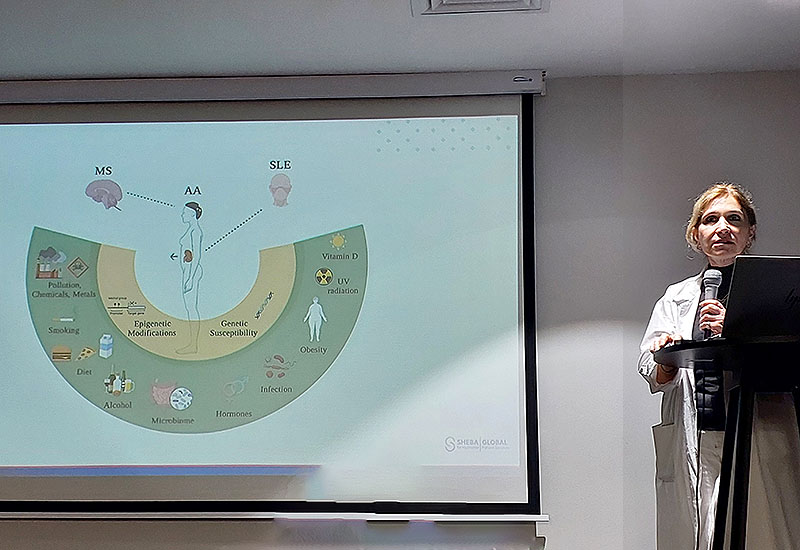
Understanding Lupus (SLE)
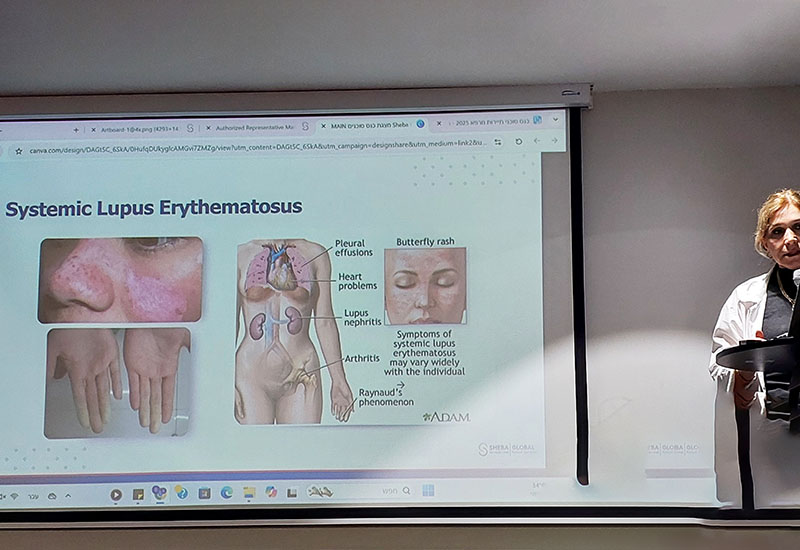
Systemic Lupus Erythematosus (SLE) — commonly known as lupus — is a chronic autoimmune disease in which the immune system becomes overactive and attacks healthy tissues throughout the body. Lupus can affect many organs, including the joints, skin, kidneys, heart, lungs, blood vessels, and nervous system.
Symptoms vary widely between patients but often include fatigue, joint pain, skin rashes, fever, and inflammation of various organs. The course of the disease is unpredictable — with periods of flares and remission — and it disproportionately affects women, especially those of childbearing age. Lupus can significantly impact a person’s quality of life, particularly when the disease does not respond to conventional therapies.
Prof. Lidar in a lecture about the CAR-T program
Traditional Treatments for Lupus – With Limitations
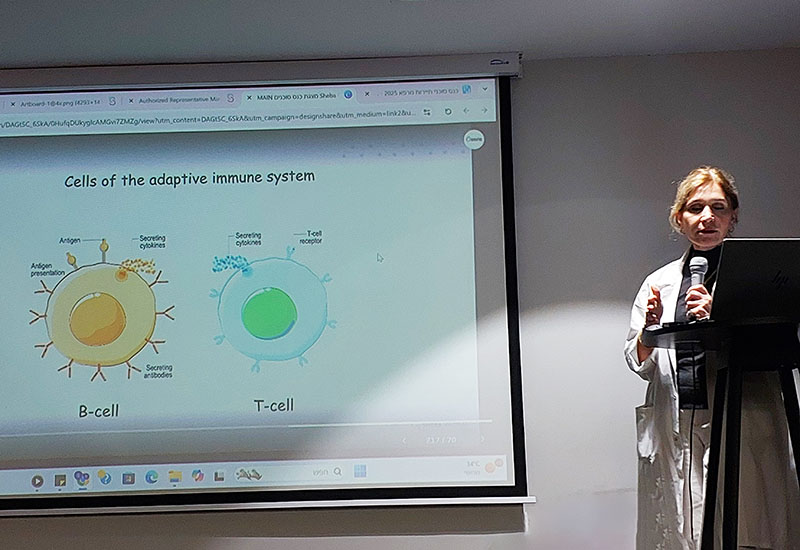
Historically, lupus has been treated with immunosuppressive medications such as corticosteroids, hydroxychloroquine, immunomodulators, and biologic drugs. One of the most widely used biologics for severe lupus is MabThera (rituximab), a monoclonal antibody that targets B-cells — the immune cells that produce autoantibodies.
Although MabThera is not officially approved for SLE in many countries, it has been used off-label for years, particularly in patients with lupus nephritis, vasculitis, or neuropsychiatric complications. Some patients respond well, while others experience limited benefits or relapse after B-cell repopulation. Overall, these treatments can help reduce inflammation and protect organ function, but they often require long-term use, bring serious side effects, and do not offer a lasting cure. For many people living with refractory lupus, the search for better solutions continues.
CAR-T Therapy: A New Era in Lupus Treatment
CAR-T cell therapy (Chimeric Antigen Receptor T-cell therapy) represents a groundbreaking approach for patients with treatment-resistant lupus. Originally developed for cancer, this form of personalized immunotherapy is now showing promising results in autoimmune diseases, including SLE. CAR-T therapy works by collecting a patient’s own T-cells, genetically modifying them in a lab to recognize specific immune targets, and reintroducing them into the body. These engineered T-cells then seek out and eliminate the malfunctioning immune cells that are driving the disease.
Why CAR-T for Lupus?
Studies and early clinical trials have shown that B-cell depletion using CAR-T cells can lead to significant and sustained remission in patients with severe lupus, and in some cases, long-term disease control approaching cure. Many patients experience dramatic improvement in symptoms, with a substantial reduction—or complete elimination—of steroid dependence.
For patients with systemic lupus erythematosus (SLE) who have exhausted conventional treatment options, CAR-T therapy offers a meaningful opportunity to regain quality of life while reducing the need for ongoing immunosuppression.
A pivotal milestone in this field was reported in 2020 by Dr. Georg Schett and his team at Friedrich-Alexander University Erlangen. They described the first successful use of CAR-T therapy in an autoimmune disease: a young woman with severe, treatment-resistant lupus received a single infusion of anti-CD19 CAR-T cells and achieved complete clinical remission within one month. More than five years later, she remains disease-free, highlighting the transformative potential of CAR-T therapy beyond oncology.
For patients who have not responded to standard therapies, CAR-T therapy represents a promising and potentially life-changing option, supported by highly encouraging early clinical outcomes.
CAR-T Clinical Trial for Lupus at Sheba Medical Center
At Sheba Medical Center in Israel, a leading clinical trial is currently underway offering CAR-T therapy for patients with systemic lupus erythematosus (SLE). This is part of a broader initiative targeting severe autoimmune and rheumatologic diseases. The program is led by Prof. Merav Lidar, a renowned expert in autoimmune conditions and Director of Sheba’s Center for Autoimmune Diseases.
Patients from around the world may be eligible to participate in this innovative program, pending medical evaluation.
To qualify, the disease must be clinically active. It is important to note that early intervention is critical — as once permanent damage has occurred in organs or tissues, it cannot be reversed. The goal is to treat the disease before irreversible harm is done.

Who May Qualify for CAR-T Therapy for Lupus?
CAR-T therapy may be considered for selected patients with systemic lupus erythematosus (SLE) who meet specific medical criteria. In general, candidates are patients with moderate to severe disease who have not responded adequately to conventional treatments and who are medically stable enough to undergo the CAR-T treatment process, which typically requires approximately 8–10 weeks in Israel.
Patient eligibility considerations include:
- Confirmed Diagnosis
A confirmed diagnosis of active systemic lupus erythematosus (SLE). - Treatment-Resistant Disease
Lupus that is refractory to standard immunosuppressive and/or biologic therapies, typically after failure of two to three prior lines of treatment. - Significant Organ Involvement
Presence of severe disease manifestations, such as lupus nephritis, neuropsychiatric lupus, or multi-system organ involvement. - Medical Stability
Clinical stability sufficient to safely undergo lymphodepletion (conditioning chemotherapy) followed by CAR-T cell infusion.
Start the Evaluation Process
If you or someone you love is living with lupus and traditional treatments have not provided relief, CAR-T therapy in Israel may offer a new path forward.
Take the first step toward hope and healing — Contact us today to learn more about this innovative treatment option.
Publication date: August 2025










