Compared with standard lupus therapies, CAR T’s main advantages are the depth of remission, the possibility of stopping other drugs, and a different, more “one off” treatment model rather than lifelong immunosuppression.
Depth and quality of remission
- Early SLE CAR T studies show very large drops in SLEDAI (for example from about 13 to about 2 at 6 months), with around 70% reaching formal DORIS remission and almost 90% low disease activity.
- Case series report complete clinical and serologic remission even in severe lupus nephritis, including normalization or major improvement of proteinuria, complements and autoantibodies, which many patients never achieve on standard drugs.
Potential for drug free control
- In several cohorts, most or all patients were able to discontinue steroids and other immunosuppressants after a single CAR T infusion, yet remained in remission for 1–3 years or longer.
- By contrast, existing options such as mycophenolate, azathioprine, belimumab, voclosporin or rituximab typically require continuous or repeated treatment, with disease flares when therapy is reduced or stopped.
Mechanistic “immune reset” vs chronic suppression
- CAR T induces profound but temporary B cell depletion and seems to “reset” the B cell compartment; when B cells repopulate, they show a more naïve phenotype and autoantibody production often stays suppressed.
- Conventional B cell–directed drugs (rituximab, anti BAFF) deplete or modulate B cells but often incompletely, and autoreactive clones can repopulate quickly, contributing to relapse despite ongoing therapy.
Advantages for severe, refractory disease
- The published CAR T lupus cohorts are almost all patients who had failed multiple standard and biologic treatments; yet remission rates (including renal responses) are higher than what is typically seen when cycling more conventional drugs in this setting.
- For some with advanced lupus nephritis, CAR T has allowed stabilization or improvement of kidney function and avoidance of dialysis, which is rarely achieved once the disease is this advanced using standard care alone.
Safety profile relative to intensity of effect
- In lupus, cytokine release syndrome has mostly been grade 1–2, with very few grade 3 events and almost no reported high grade neurotoxicity, which is substantially milder than in oncology CAR T experience.
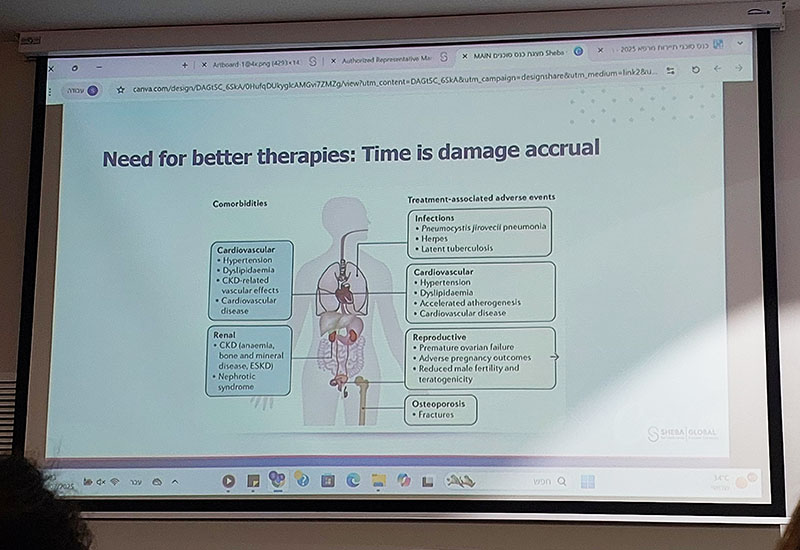
- Traditional immunosuppression carries cumulative risks of infection, malignancy, cardiovascular disease and steroid damage over many years; CAR T concentrates risk into a short intensive period, with the hope of minimizing long term exposure to these chronic toxicities.
Read more CAR-T patient testimonials >
Publication date: Feb 2026.
Sources:
*emjreviews.com: New Evidence Supports CAR-T Cells as a Potential Breakthrough in SLE Care
*pmc: CAR-T cell therapy: A new dawn in the treatment of autoimmune disease
*pmc: CAR T-cell therapy for systemic lupus erythematosus: current status and future perspectives
*pmc: CAR-T-Cell Therapy for Systemic Lupus Erythematosus: A Comprehensive Overview
*rheumatology.org: CAR-T Cell Therapies Show Promise for Autoimmune Disease at ACR Convergence 2025
*ucdavis: A breakthrough for lupus treatment? Study explores CAR T-cell therapy for autoimmune disease
*clinicbarcelona.org: CAR T-cell therapy: a new treatment option for autoimmune diseases
*lupus.org: Data Suggests CAR-T Therapy Shows Promise for Lupus Treatment
CAR-T for Lupus in Israel
Find out if CAR-T cell therapy can be suitable for you >










