Hadassah Hospital Homegrown CAR-T Therapy for Multiple Myeloma
Hadassah Hospital in Israel began developing its homegrown academic CAR-T therapy in 2017, the first treatments in patients begun in 2021, and as of 2023, more than 80 multiple myeloma patients were treated with remarkable results.
The version of this treatment for multiple myeloma patients was developed by professors Polina Stepenksy and Cyril Cohen. Stepensky is the director of the Bone Marrow Transplantation and Immunotherapy Department at Hadassah, and Cohen is the head of the Cancer Immunology and Immunotherapy Laboratory at the Faculty of Life Sciences at Bar Ilan University.
This technology is approved by the FDA for multiple myeloma patients. The therapy is part of a clinical study approved by the Helsinki Committee and the Israeli Ministry of Health.
Hadassah Hospital’s CAR-T therapy program was achieved by cooperation with NexImmune, an American biotechnology company that specializes in immune-oncology therapies. Together they created their own patented CAR-T treatment called NXC-201 CAR-T. Read more >>
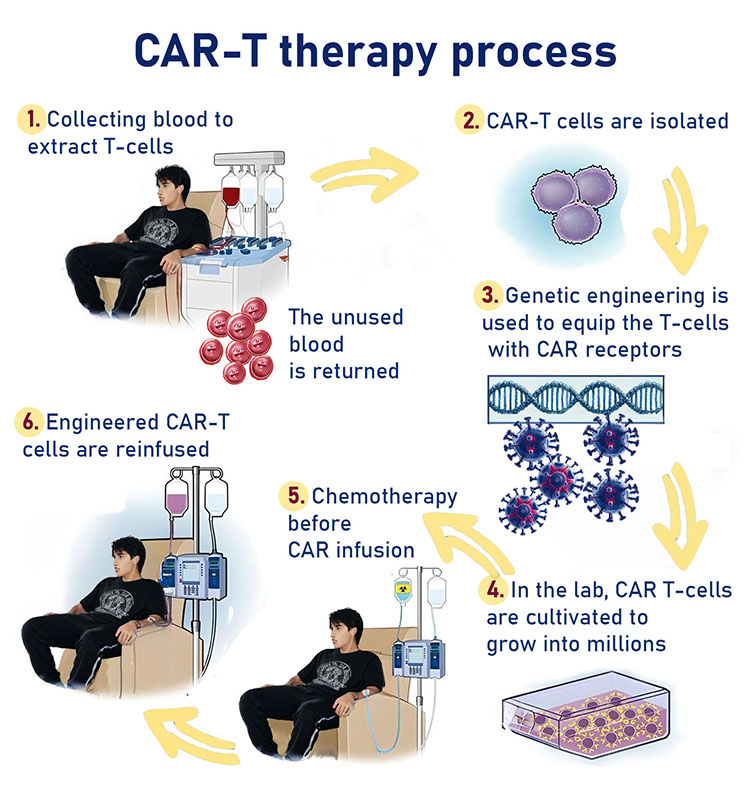
As part of the treatment, researchers isolate T cells from the patient's immune system. The cells are then genetically engineered in a laboratory so that they express a receptor to identify the cancer cell. This receptor is a protein in the cell whose function is to receive information that includes operating instructions such as when to divide or self-destruct. In the next step, a large amount of the engineered cells are grown in a laboratory and then injected into the patient. The receptor on the T cells binds to the protein that characterizes the cancer cell so the T cell can attack and destroy it.
The treatment is a one-time infusion.
The cost of the CAR-T is significantly lower than the price of the commercial CAR-T’s.
SUCCESS RATE
The success rate in the 50-plus patients treated so far is 90 % percent. The ages of the patients were 44-84, and the success rate was not affected by age. The treatment is a game changer for Multiple Myeloma patients.
CRITERIA FOR ELIGIBILITY FOR INTERNATIONAL PATIENTS
The patient must have treatment-resistant multiple myeloma, with no remission after three different and unsuccessful lines of therapy. (Imids, proteasome inhibitors and anti CD 38)
STANDARD COURSE OF TREATMENT FOR INTERNATIONAL PATIENTS
- Send medical documentation for a free evaluation to see if the patient is eligible for the treatment.
- Receive a remote consultation with Prof. Stepensky.
- Receive a treatment plan and cost estimate.
- Arrive at Hadassah hospital. Perform diagnostics and evaluation—about one week. (Ambulatory)
- Collection of T-cells and 10 days of Laboratory work. Hospitalization begins. Patient receives light chemotherapy as preparation for the CAR-T cells infusion.
- Receive the infusion of CAR-T and remain as an inpatient for two-weeks to manage side effects. The patient stays in isolation with only the person who arrived as their escort during the treatment.
- 2 weeks of stay in Israel, arrive for ambulatory treatment if needed. International patients who wish to fly back to their country and return for PET-CT can do so.
- Undergo a PET CT to evaluate the success of the treatment (One month after the infusion of the T-cells).
- Continue follow up checkups in your home country.
- Total estimated stay in Israel for the patient and escort- 1 week + 10 days + 1 month = 47 days

Professor Polina Stepenksy (Read more >>)
SIDE EFFECTS
Side effects may occur on the day of the infusion or a few days afterward. Side effects are actually welcomed because they mean that the body is responding to the treatment. The side effects are easy to manage, and they might include a rise in body temperature, low blood pressure, or low urine output.
CAR-T side effects safety statistics so far:
- There was no irreversible damage to the organs, there was no mortality
- Most of the patients had CRS, almost all grade 1-2, and in a few patients grade 3
- A need for "Tocilizumab" was common.
- There was one and only case of ICANS (neurological toxicity), grade 1
Testimonial by a CAR-T patient at Hadassah hospital

My Husband and I engaged with Tanya Preminger from IHA to travel from New Zealand to Israel for undertaking Car T treatment. During the preparation stage Tanya was very professional in her approach and extremely responsive to our many emails. During our 8 week stay in Israel Tanya was incredibly supportive on both a practical and social level. Tanya was an effective communicator with ourselves and the Hadassah hospital staff, always accountable and flexible with our needs. We would highly recommend Tanya as a representative for other clients who may be considering various treatments in Israel.
Yvonne and Mike Hogan, New Zealand, 8/5/2023

Further reading:
- More about the CAR T program in Hadassah
- About Hadassah Hospital
- About Prof. Polina Stepenksy
- Interview with Prof. Stepenksy
- Advancements in CAR-T Therapy for Multiple Myeloma at Hadassah Medical Centre 2023

Prof Stepensky and our CAR-T specialist, Tanya
Links
- CAR-T therapy offers hope in Hadassah
- Article in “Ha’aretz” – Israeli newspaper: Israeli Experimental Treatment for Multiple Myeloma Shows Promising Results. May 2023.
- Nexcella, an Immix Biopharma Subsidiary, Announces New Positive NXC-201 Clinical Data Demonstrating 100% Complete Responses in AL Amyloidosis Patients + Additional Positive Multiple Myeloma Safety Data. Read >>
- NXC-201 Continues to Show Safety, Efficacy in Relapsed/Refractory Multiple Myeloma
- Israeli CAR-T cancer treatment could be administered out-patient - first in world - An article in the Jerusalem post, March 2023
- Hadassah develops its own CAR T therapy to treat Multiple Myeloma
- Newsweek Survey Ranks Hadassah Among Top Hospitals in Oncology, Cardiology and Smart Technology(March 2023)
- Jerusalem Post-Blue-and-white CAR T in Israel










